Hydrogen is increasingly seen as a vital component for eliminating or at least reducing emissions from ‘hard to decarbonise’ sectors, such as heavy duty transport, domestic and commercial heating, and carbon-intensive industrial processes (concrete, steel, food & drink, etc.). Whilst using hydrogen as a fuel is zero-emission at its point of operation, with only water as its product, its overall well-to-wheel carbon footprint is largely determined by its method of production. Indeed, when derived from fossil fuels, over its lifecycle it may have worse greenhouse gas (GHG) emissions than if one were to use those fossil fuels directly.
carbon-intensive industrial processes (concrete, steel, food & drink, etc.). Whilst using hydrogen as a fuel is zero-emission at its point of operation, with only water as its product, its overall well-to-wheel carbon footprint is largely determined by its method of production. Indeed, when derived from fossil fuels, over its lifecycle it may have worse greenhouse gas (GHG) emissions than if one were to use those fossil fuels directly.
To understand the impact of hydrogen production methods and their embedded GHG emissions, the hydrogen colour palette was devised as a means to distinguish between carbon intense and low or effectively zero carbon sources of hydrogen.
Green Hydrogen
Utilising surplus renewable electricity to electrolyse water is a method which is truly zero-carbon from an operational point of view and its product is therefore coined ‘green hydrogen’. Its only by-product is oxygen, which is usually vented to atmosphere, but may be collected as it has economical value for instance in medical use or wastewater treatment plants. The UK and Scotland, in particular, are ideally suited to be producers of green hydrogen, thanks to an abundance of renewable energy sources and relatively small domestic electricity demand from its small population. Production of green hydrogen is thus actively pursued by the Scottish Government as a vehicle to decarbonise various sectors and as a potential export product. It is also integral to Scotland’s ‘Just Transition’, utilising existing skills and expertise from the oil & gas industry to create green jobs for the renewable sector. A lifecycle analysis suggest that the well-to-wheel GHG emissions are currently lowest for hydropower based electrolysis (0.3 kg CO2e kg H2), followed by wind (0.5) and solar (1.0), due to emissions associated with manufacture of turbines, solar panels, etc. [1]
operational point of view and its product is therefore coined ‘green hydrogen’. Its only by-product is oxygen, which is usually vented to atmosphere, but may be collected as it has economical value for instance in medical use or wastewater treatment plants. The UK and Scotland, in particular, are ideally suited to be producers of green hydrogen, thanks to an abundance of renewable energy sources and relatively small domestic electricity demand from its small population. Production of green hydrogen is thus actively pursued by the Scottish Government as a vehicle to decarbonise various sectors and as a potential export product. It is also integral to Scotland’s ‘Just Transition’, utilising existing skills and expertise from the oil & gas industry to create green jobs for the renewable sector. A lifecycle analysis suggest that the well-to-wheel GHG emissions are currently lowest for hydropower based electrolysis (0.3 kg CO2e kg H2), followed by wind (0.5) and solar (1.0), due to emissions associated with manufacture of turbines, solar panels, etc. [1]
Grey Hydrogen
Currently, the vast majority (around 95%) of the world’s hydrogen is derived from natural gas, through a process called steam methane reforming (SMR). This process combines natural gas (essentially methane) and steam to produce syngas, a combination of carbon monoxide and hydrogen, see reaction 1. The carbon monoxide can then be converted to yield additional hydrogen via the water-gas shift reaction (2), which also generates a molecule of carbon dioxide, CO2. The process is endothermic, so requires heat provided by burning fossil fuels, leading to additional carbon emissions. Further GHG emissions are caused by accidental leakages of methane upstream, which can be significant, particularly when considering methane’s potent GHG effect, around 40 times that of CO2.
process called steam methane reforming (SMR). This process combines natural gas (essentially methane) and steam to produce syngas, a combination of carbon monoxide and hydrogen, see reaction 1. The carbon monoxide can then be converted to yield additional hydrogen via the water-gas shift reaction (2), which also generates a molecule of carbon dioxide, CO2. The process is endothermic, so requires heat provided by burning fossil fuels, leading to additional carbon emissions. Further GHG emissions are caused by accidental leakages of methane upstream, which can be significant, particularly when considering methane’s potent GHG effect, around 40 times that of CO2.

A related process, auto-thermal steam reforming (ATR), combines partial oxidation and SMR and leads to a similar amount of CO2 released as part of the chemical process (reaction 3). However, it is significantly less endothermic and thus result in slightly reduced carbon emissions from process heating. Overall GHG emissions (expressed in kg CO2 equivalent) for SMR and ATR are 9.2 – 11 kg CO2e / kg H2.[1]
Blue Hydrogen
Blue hydrogen is essentially identical to grey hydrogen, with the exception that carbon capture, (utilisation) and storage (CCS or CCUS) is applied post-process to reduce CO2 emissions. High rates of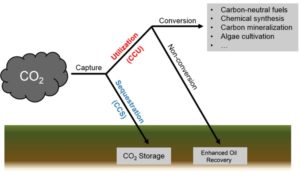 CCS can only be achieved if post combustion CO2 is also captured. This is more difficult than capturing reaction CO2 and SMR can therefore only achieve around 90% capture, whereas ATR is reported to be capable of reaching 98% capture rates. These rates do not include emissions arising from the CCS process itself and accidental methane leakages still contribute to GHG emissions as well. The ultimate capture rates are also likely to be determined by economics as opposed to technical feasibility and as such will be driven by government policy. A best-case scenario for emissions from ‘blue hydrogen’ still results in 1.5 – 3.9 kg CO2e / kg H2.[1]
CCS can only be achieved if post combustion CO2 is also captured. This is more difficult than capturing reaction CO2 and SMR can therefore only achieve around 90% capture, whereas ATR is reported to be capable of reaching 98% capture rates. These rates do not include emissions arising from the CCS process itself and accidental methane leakages still contribute to GHG emissions as well. The ultimate capture rates are also likely to be determined by economics as opposed to technical feasibility and as such will be driven by government policy. A best-case scenario for emissions from ‘blue hydrogen’ still results in 1.5 – 3.9 kg CO2e / kg H2.[1]
Turquoise Hydrogen
Turquoise hydrogen is another form of hydrogen production which relies on natural gas, but the process generates solid carbon black, as opposed to CO2, as a by-product and as such is a low carbon production method. Production at scale is now underway at a select few sites globally, but the process is still considered a niche approach. Continued emissions from methane leakages still pose a problem as well.
Yellow Hydrogen
Essentially identical to green hydrogen, but electricity for the water electrolysis process is provided by surplus nuclear energy. This is potentially less relevant for Scotland, but more so for the UK. Operational GHG emissions are only those associated with mining and processing nuclear fuels. The issue of nuclear waste is an additional environmental as well as political concern.
Black Hydrogen
One of the oldest and most carbon intensive methods of producing hydrogen is through coal gasification, where coal is combined with controlled amounts of oxygen and/or steam to produce a carbon monoxide rich syngas at temperatures of around 700°C, see reaction 4. As for SMR and ATR, additional hydrogen is produced via the water-gas shift reaction (2) also producing CO2. The overall process is exothermic. GHG emissions from the process alone amount to 17 kg CO2e / kg H2. The terminology black versus brown hydrogen refers to the source of coal: brown and black coal, or lignite and bituminous, respectively.
gasification, where coal is combined with controlled amounts of oxygen and/or steam to produce a carbon monoxide rich syngas at temperatures of around 700°C, see reaction 4. As for SMR and ATR, additional hydrogen is produced via the water-gas shift reaction (2) also producing CO2. The overall process is exothermic. GHG emissions from the process alone amount to 17 kg CO2e / kg H2. The terminology black versus brown hydrogen refers to the source of coal: brown and black coal, or lignite and bituminous, respectively.
| 4 |
[1] Hydrogen decarbonization pathways, A life-cycle assessment, January 2021, www.hydrogencouncil.com